Understanding Single Crystal X Ray Crystallography
❤️ Click here: Understanding single-crystal x-ray crystallography pdf
Author : Anette Müllertz ISBN : 9781493940295 Genre : Medical File Size : 31. A full data set may consist of hundreds of separate images taken at different orientations of the crystal. In other words, using a crystal concentrates the weak scattering of the individual unit cells into a much more powerful, coherent reflection that can be observed above the noise. However, as computational and experimental methods improved over the next decades, it became feasible to deduce reliable atomic positions for more complicated two- and three-dimensional arrangements of atoms in the unit-cell.

Here we consider thick symmetrical specimens. Finally, X-ray crystallography had a pioneering role in the development of , particularly in clarifying the structures of the and the principles of. Any sufficiently thick crystal will produce secondary scattering, but since X-rays interact relatively weakly with the electrons, this is generally not a significant concern.

Understanding Single Crystal X Ray Crystallography - X-ray crystallography is now used routinely by scientists to determine how a pharmaceutical drug interacts with its protein target and what changes might improve it.
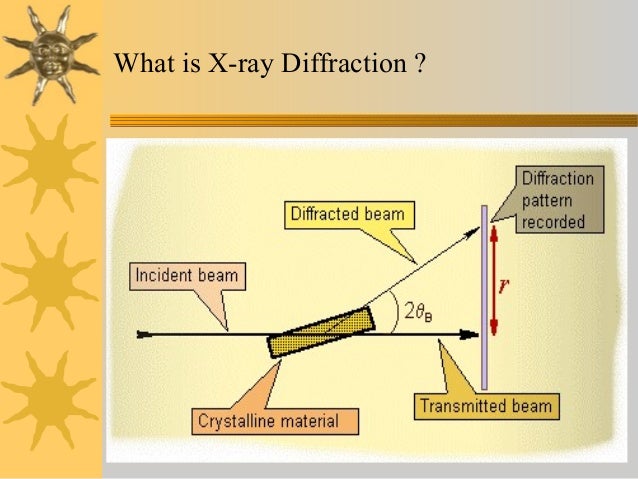
Brenner, in , 2001 X-ray crystallography is a technique for determining the three-dimensional structure of molecules, including complex biological macromolecules such as proteins and nucleic acids. It is a powerful tool in the elucidation of the three-dimensional structure of a molecule at atomic resolution. Data is collected by diffracting X-rays from a single crystal, which has an ordered, regularly repeating arrangement of atoms. Based on the diffraction pattern obtained from X-ray scattering off the periodic assembly of molecules or atoms in the crystal, the electron density can be reconstructed. Stubbs II, in , 2007 3. Advances in scientific instrumentation, x-ray sources and detectors, computing, and in particular molecular biology have lead to a situation where experimental three-dimensional structure data of a drug target are no longer an academic dream but a realistic goal. It is the aim of this chapter to provide a theoretical and practical background to this important method, which is based on my lecture course and recurring questions raised by my graduate students. While it has now become possible to solve a protein crystal structure without any knowledge of the underlying principles, crystallographic theory is ignored at the risk of failure to elucidate important structural details if problems occur. In this short chapter, it is not possible to cover all details pertaining to x-ray crystallography; for these, the interested reader is directed to Rossmann and Arnold's excellent recent reference work. Although generally accepted as being electromagnetic in nature, no direct proof could be found, as the rays failed to be refracted in any measurable sense. Working in Munich in the department of the theoretician Sommerfeld, Max von Laue 1879—1960 postulated that if x-rays were electromagnetic, then they should be of extremely short wavelength. If the wavelength was of the order of interatomic distances, then a crystal should act as a three-dimensional diffraction grating for x-rays. Bragg championed x-ray methods for the study of living systems. Although Sumner, Northrop, and Stanley had shown that enzymes and viruses could be crystallized an achievement for which they were awarded the Nobel Prize in Chemistry in 1946 , all attempts to obtain diffraction images from protein crystals failed. A decisive breakthrough was made in Cambridge in 1934, when J. Bernal 1901—1971 and Dorothy Crowfoot Hodgkin 1910—1994 noted that crystals of pepsin lose their birefringence on exposure to air. Although this was at a time when even the structure determination of molecules with only few atoms was a major undertaking, the way was paved for the use of x-ray analysis to explore biological macromolecules at the atomic level. Working initially in Bragg's laboratory in the Royal Institution in London and then later in Leeds, W. Astbury 1889—1961 obtained fiber diffraction patterns from keratin as well as a number of other biological specimens. Keratin diffraction patterns measured at varying humidity indicated two distinct, characteristic spacings corresponding to 5. Based on precise measurements concerning the stereochemistry of amino acids, in particular the planarity of the peptide bond, Linus Pauling 1901—1994 of CalTech was able to explain Astbury's observations by proposing models for the α-helix and the β-sheet. Upon hearing of Pauling's model, Max F. Perutz 1914—2002 , who had been working on hemoglobin crystals in Bragg's laboratory in Cambridge since 1937, made x-ray measurements from his crystal data that confirmed the existence of the α-helix. Crick 1916—2004 the task of providing a mathematical treatment for the diffraction from a helix. Watson 1928— , culminating in the proposal of a double helical structure for DNA, 10 has been well documented, as well as the role played by the x-ray fiber diffraction patterns of Rosalind Franklin 1920—1958 11 and Maurice Wilkins 1916—. While the intensities of the individual reflections in the diffraction pattern could be measured with some accuracy, the vital information for calculating an electron density, the phase information, was missing see Section 3. Perutz suggested that the introduction of heavy atoms into the large solvent channels of hemoglobin crystals could give rise to measurable changes in the diffraction pattern, which could in turn be used to solve the phase problem. As the number of structures solved increased, so it became necessary to catalog and characterize them. Thus the Protein Data Bank PDB was born, 17,18 a depository for the coordinates of all published structures. The realization that many structures possess similar folds lead to a new method for structure determination: molecular replacement or the Patterson search method. Yet there was one class of proteins whose structure determination remained elusive: the membrane proteins. The first structure of an integral membrane protein was revealed in 1985: that of the bacterial photosynthetic reaction center. In part for his work on the structure determination of ATP synthase, 23 John E. Protein crystallography has progressed from a discipline driven by technology to a field driven by biological, physiological, or pharmaceutical questions. The recent structure determinations of ribosomes and their subunits 25—27 show that even vast multimolecular complexes are amenable to crystallographic analysis, and have provided detailed information as to the workings of, e. This resource provides a basis for theoretical analyses of biological macromolecular structure. Experience with experimental structures shows, however, that at least for the time being, our theories are not sufficiently advanced to describe the intricacies of biology in detail at an atomic level. For this, it will remain necessary to probe the nature of proteins using experimental biophysical methods; the aim of this chapter is to provide an understanding for the processes involved in protein crystallography. Moody, in , 2011 13. Thus thick specimens are associated with either low resolution, or increased complexity of data-collection and processing. Here we consider thick symmetrical specimens. Every translation leads to an extension of at least tens of unit cells along the translation. Three independent translations thus imply a specimen of at least tens of unit cells in every dimension. This usually leads to a specimen too thick to transmit electrons of the voltages used in most electron microscopes 100—300 kV. There are two solutions to this problem: reducing the expected thickness by sectioning, and increasing the voltage much further. The latter possibility is not only very expensive, but also faces interference and other problems with thick specimens Chapter 15 of Glaeser et al. The process of cutting thin sections reduces the resolution to a pre-molecular level. That is nevertheless still most informative in the case of muscle, and there has been considerable study of well-ordered muscles by thin-sectioning. The most obvious defect of sectioning is to remove parts of the lattice outside the section, and the need to restore this has led to ingenious computational methods 1. Very high voltage microscopes exist in special locations, but they appear not to have been much used for forming images of 3D lattices. Instead, the study of these has been the virtual monopoly of X-ray crystallography. The following brief and fairly superficial survey of this large and complex subject is intended to indicate how X-ray diffraction fits into a general pattern of diffraction-based structural techniques, and also to serve as an elementary introduction to newcomers. Those interested in going further, though without getting too deep, can consult Blow's general introduction for biologists Blow, 2002 , or especially for those needing to use crystallographic structures Rhodes, 2006. There are also many more thorough though mathematical textbooks, including Giacovazzo 1992 especially for general crystallography and for protein crystallography the new book by Rupp 2010. Finally, it must be possible to extract the specimen structure from the changes in transmitted radiation. In view of the proverbial penetration of X-rays into ordinary matter, it might seem surprising that considerable work was needed to explain how X-rays are diffracted by ordinary crystals without attenuation, that would be expected from the scattering power of most atoms. This is only possible because the lattices aren't perfect, and the imperfections lead to small regions diffracting at closely similar, but not quite identical, angles. This is just one of the remarkable pieces of good fortune that, together, eventually made possible the early crystallographic study of protein structure, using millimeter-sized crystals of relatively small proteins. However, more intense X-ray sources allow the study of much smaller crystals less than 50 μm thick , comparable in size to the ordered regions themselves. Interaction with the specimen, without destroying it, is really one problem: how much information is obtainable before specimen-destruction is becoming unacceptable? This is the problem of radiation-damage considered below §13. However, for many years the overwhelming problem was that of extracting the specimen structure. Then Perutz's discovery 2 of the applicability of the isomorphous replacement method Bokhoven et al. However, structurally related proteins were soon found especially the globins. Obviously at first most structures were completely novel, so the ab initio methods were essential. Its relative importance will presumably continue to grow, as a more comprehensive knowledge of protein structures or at least of domains is gradually acquired. Thus there are two fundamentally different approaches to solving protein structures: ab initio methods based on adding a very few strongly-scattering centers, and molecular replacement methods based on information from similar known structures. However, most of the experimental techniques are common to both methods, and they are described first. Abraham, in , 2007 4. However, in the 1980s Ringe and Petsko 177 studied protein dynamics by x-ray crystallography and Hajdu and colleagues used Laue diffraction that is capable of recording entire protein crystal data sets in a millisecond. With this development it will be possible for medicinal chemists to use SBDD to discover transition-stated analogs with a probable higher rate of success than with the static receptor design methods. This technique might also be valuable in assaying flexible binding sites during the initial binding of inhibitors or substrates. X-ray diffraction can generate limited high resolution structural information, but only for membranes that have highly ordered crystalline repeating units. A few biological membranes eg, myelin sheath and the rod outer segment are naturally stacked and so are ideally suited for X-ray diffraction studies. In fact as early as 1935, X-ray studies on myelin were consistent with the presence of a lipid bilayer. In addition, mitochondria and erythrocyte membrane vesicle preparations and phospholipid vesicles liposomes can be collapsed by centrifugation, also making them suitable for X-ray diffraction. The electron density profiles of all membranes look very similar by X-ray diffraction. They consist of a low electron density hydrocarbon interior flanked by a high electron density polar group on either side. This bilayer has a 43 Å separation between polar head groups. The addition of more lipids or even proteins only slightly modify the profile. Details of membrane protein structure, for example, cannot be obtained by X-ray diffraction. Therefore, X-ray diffraction does not yield molecular detail of membrane structure, but does support the concept of a lipid bilayer. Electron density profiles obtained by X-ray diffraction for lipid vesicles liposomes made from DMPC solid line or DMPC—cholesterol 0. The most electron-dense part of the spectrum corresponds to the polar head group regions. X-ray crystallography is a tool used for determining the atomic and molecular structure of a crystal. The underlying principle is that the crystalline atoms cause a beam of X-rays to diffract into many specific directions Fig. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a 3D picture of the density of electrons within the crystal. From this electron density image, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their disorder, and various other information. The method revealed the structure and function of many biological molecules, including vitamins, drugs, proteins, and nucleic acids, such as DNA. Note that the double helix structure of DNA discovered by James Watson and Francis Crick was revealed by X-ray crystallography. Recent advances in image reconstruction technology have made X-ray crystallography amenable to the structural analysis of much larger complexes, such as virus particles Fig. The major shortcoming of X-ray crystallography is that it is difficult to obtain a crystal of virus particles, which is a prerequisite for X-ray crystallography. Another shortcoming is that X-ray crystallography generally requires placing the samples in nonphysiological environments, which can occasionally lead to functionally irrelevant conformational changes. Jean-Michel Rondeau, Herman Schreuder, in , 2015 Protein crystallography is an integral part of pharmaceutical research. On-going developments in miniaturization, robotics, etc. Often, crystal structures will already be available from the public domain at the start of a project. Here we discuss all aspects of protein crystallography within drug discovery, starting with a historic overview, followed by the crystallization of proteins, with emphasis on requirements for crystallizable proteins and the preparation of protein—ligand complexes. Also, specific issues and tricks for protein families like proteases, kinases, and G-protein-coupled receptors are discussed. The theoretical background of crystallography and the effect of disorder are briefly discussed, so that medicinal chemists and modelers gain a better understanding of the strengths and limitations of protein crystallography. The second part discusses the application of protein crystallography in the drug discovery value chain, especially fragment-based screening, hit selection, and lead optimization, where historically crystallography had its largest impact. Ueli Aebi, in , 2004 Publisher Summary X-ray crystallography is one of the few experimental methods that makes possible to study intermediate filament IF structure at atomic resolution; however, the prerequisite for a crystallographic analysis is the ability to produce macroscopic, well-ordered crystals. This chapter focuses on the existing experience with x-ray crystallography. It also discusses another diffraction method—small angle x-ray scattering SAXS —which is used to investigate IF structure at higher assembly levels than the dimer. It outlines the approaches that bring together data on the IF protein structure obtained by various methods, aiming at constructing the three-dimensional model of complete IFs—a goal that has not been achieved yet. Although, the structural resolution provided by SAXS is less than that of x-ray crystallography, the advantage of SAXS is that it works on protein solutions and, hence, does not depend on the availability of crystals. Moreover, SAXS allows the monitoring of the consequent steps of IF assembly by varying the solution conditions and provides additional information on the assembly pathway. The crystallization of stable complexes formed by various IF fragments is a difficult task; however, this should provide the essential atomic detail on the four dimer—dimer contact types occurring in mature IFs. Both x-ray crystallography and SAXS have a large future potential toward getting more structural insight into the interactions of the elementary IF dimers during assembly. Bond distances Å C 1 —C 2 1. The X-ray crystal structures of the interesting phosphonium azoniaspiroylides 7 and 8 have been published. The structures were originally deduced from the 1H and 13C spectroscopy data, in particular the chemical shifts of the protons and carbons adjacent to the quaternary nitrogen atom, but were confirmed by the X-ray crystallographic structures.
Single crystal x-ray diffraction
The oscillations carried out during data collection understanding single-crystal x-ray crystallography pdf below involve the ω axis only. Calculating Structure Factors and Electron Density 257 4 Crystal Diffraction: Experiment 265 4. The most electron-dense part of the spectrum corresponds to the polar head group regions. North Refinement 710 8. For all above mentioned X-ray diffraction methods, the scattering is ; the scattered X-rays have the same as the incoming X-ray. On-going developments in miniaturization, robotics, etc. Moody, in2011 13. In order to obtain an interpretable electron density map, both resistance and phase must be known an electron density map allows a crystallographer to build a starting model of the molecule. This means that 10-fold less protein is used per experiment when compared to crystallization trials set up by hand in the order of 1.



